Bharat Parenterals – Walk-In Interviews for Production / QA / F&D / Purchase / Artwork / Tablet Capsule/ BSR on 23rd & 25th Dec’ 2023
Bharat Parenterals – Walk-In Interviews for Production / QA / F&D / Purchase / Artwork / Tablet Capsule/ BSR on 23rd & 25th Dec’ 2023
Bharat Parenterals Limited is a WHO GMP & an ISO 10002: 2014, 9001: 2015 certified pharmaceutical company, Bharat Parenterals Ltd has traversed a long way in order to be recognized as one of the fastest growing Pharmaceutical Companies in India today. Bharat Parenterals Ltd. is a Gujarat based pharmaceutical company, established in 1992 by Mr. Ramesh Desai, who started the company with a vision of making world class affordable medicines and to take it to the forefront of contract manufacturing units in Gujarat
Looking for a new career opportunity? Join us for our Walk-In Interview and take the first step towards your dream job. We’re seeking talented individuals who are passionate about pharmaceuticals to join our team.
JOB DESCRIPTION:
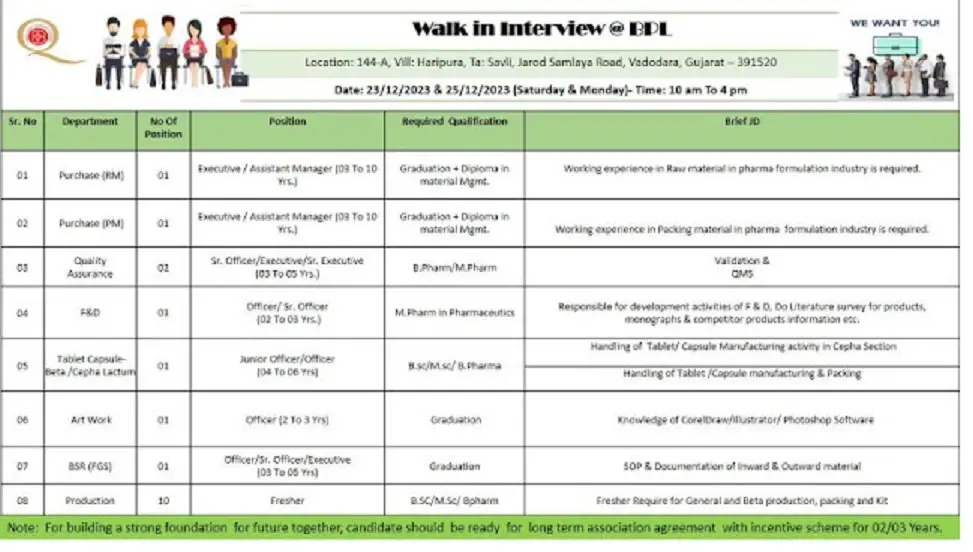
1) Department: Production / QA / F&D / Purchase / Artwork / Tablet Capsule/ BSR
·
Position: Officer – Executive / Assistant Manager
. Qualification: B.Sc / M.Sc/ B.Pharm / M Pharm/ Any Graduation / Diploma
. Experience: 02-10 yrs / Freshers
.No. of Vacancy: 18
Job Location: Vadodara
Date: 23rd and 25th December 2023
Time: 10:00AM TO 4:00PM
Venue- 144-A, Village; Haripura, Ta: Savli, Jarod Samlaya Road, Vadodara- Gujarat 391520
Come prepared to showcase your skills, experience, and enthusiasm. This is your chance to make a lasting impression and land your dream job. Don’t miss out!