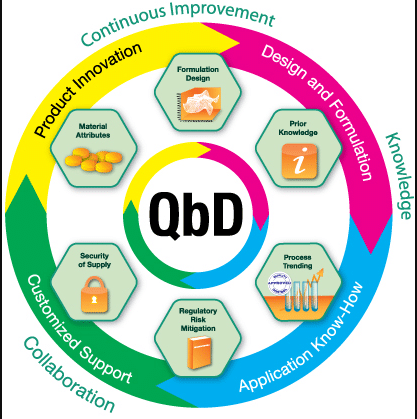
Manufacture of the finished Dosage Form – QbD: New EMA Guideline
Manufacture of the finished Dosage Form – QbD: New EMA Guideline The European Medicines Agency (EMA) published its new guideline on Manufacture of the finished dosage form on August 14, 2017. The guideline replaces the “Note for Guidance on Manufacture of the Finished Dosage Form” (dated April 1996) and will enter into effect on February 14, 2018. … Read more