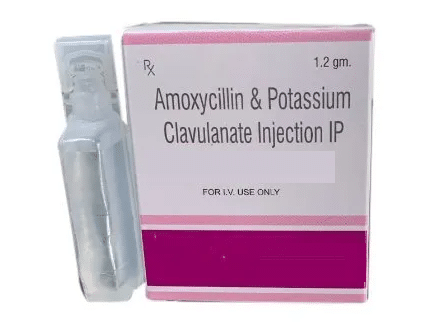
AMOXYCILLIN CLAVULANATE FOR INJECTION
AMOXYCILLIN CLAVULANATE FOR INJECTION PRODUCT INTRODUCTION AMOXYCILLIN- CLAVULANATE FOR INJECTION is a penicillin-type of antibiotic that helps your body fight infections caused by bacteria. It is used to treat infections of the lungs (e.g., pneumonia), ear, nasal sinus, urinary tract, skin, and soft tissue. It will not work for viral infections such as the common … Read more