Compressed air validation in Pharmaceutical Industries
Compressed air is air under pressure above atmospheric pressure. Useful for many household and industrial purposes. Compressed air is an important source of energy and is used in a variety of industrial applications. When properly processed, compressed air is considered safe and clean compared to other energy sources. The compaction provides the power source for pneumatic conveyors that transport liquids, powders, and moisture-sensitive products throughout the plant.
Understand the importance of product risk by monitoring and testing air against quality standards, providing accurate results, and identifying risk factors. Testing and monitoring of compressed air and other process gases (such as nitrogen, oxygen, argon, and carbon dioxide) that come into direct contact with pharmaceutical products is necessary to ensure the quality and safety of pharmaceutical products. Compressed air testing is a critical element of pharmaceutical production and affects the quality of the final product. It is used by workers in many industries, including oil and gas, pharmaceutical, manufacturing, and medical. Compressing ambient air concentrates common contaminants already present in the air and can introduce other contaminants such as oil vapors, condensation and bacteria.
Compressed air in some industries requires very high-quality air, as well as breathing air testing, instrument air testing, and nitrogen gas testing.
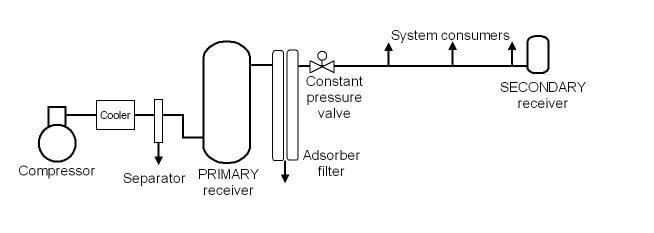
Compressed air System Description:
The air compressor unit supplies unlubricated compressed air taken from the atmosphere through the suction filter and its various components at the discharge end, which is dried, stored, filtered, and finally supplied to the service valve. The system consists of:
Air suction and filtration
Water-cooled heat exchanger
compressor motor
oil pump
safety valve
Air Dryer
air receiver tank
mist separator
The smell can be removed
FRL block (filter and regulator)

Planning and qualification methodology:
Testing and monitoring of compressed air from compressed air manifolds and critical user points in direct contact with the product is necessary to ensure product quality and safety. Compressed air is a critical process parameter (CPP) whose variability affects critical quality attributes (CQAs) and must be monitored or controlled to ensure the process is delivering the desired quality.
During testing on the compressed air manifold, the following test parameters must be performed:
number of particles
carbon dioxide
Carbon monoxide
sulfur dioxide
Nitric oxide
nitrogen dioxide
water content
Dew point
oil mist
During verification, the following testing parameters must be performed from critical user points:
Check the dew point.
Checking the water vapor content.
Checking the oil mist content.
Number of non-viable particles.
The total number of viable bacteria at each identified location is monitored continuously for 3 days.
Compressed air quality:
The quality of compressed air is important to ensure product safety. The most important parameters when determining the quality of compressed air are:
• Dew point
• Oil content
• Particulate matter
• Moisture contents
• Number of survivors.
The International Organization for Standardization has established the compressed air quality standard ISO:8573.
Dew point
Dew point temperature or saturation temperature can be defined as the temperature at which water vapor begins to condense. The amount of gas in a mixture can be expressed as pressure. The main components of compressed air are nitrogen, oxygen, and water vapor, so the total atmospheric pressure is the sum of the partial pressures of these three gases. While nitrogen and oxygen exist in stable concentrations, water vapor concentrations vary greatly and must be determined by measurement.
A gas containing an unknown concentration of water vapor is passed over a temperature-controlled surface. The surface is cooled until condensation forms. The temperature at which condensation occurs is called the “dew point temperature.” Because of the unique correlation between temperature and vapor pressure, measuring the dew point temperature of a gas is a direct measurement of the partial pressure of water vapor. Desiccant dehumidifier systems absorb water vapor from the air stream and can produce drier air with a -40°F (-40°C) dew point when needed. The dew point temperature of compressed air can vary from ambient temperature to -112°F (-8°C), but may be lower in special cases.
Oil content:
The compressed air oil content test measures the oil content of a compressed air system. This test accurately measures the amount of oil entering the compressed air stream and evaluates whether the system meets the relevant requirements. Many compressed air systems come with a lubricated compressor. These compressors often carry small oil particles throughout the system, and when the oil emulsifies with the water in the system, it can cause a variety of component problems. Compressed air systems typically require oil-free air, with an oil content typically less than 0.01 mg/m3.
Particulate matter:
Compressed air is used in a wide variety of industries, including mixing materials, cutting, sparging, drying products, conveying/moving products through processing systems, and packaging finished products. In many of these applications, compressed air comes into direct or indirect contact with the product, so impurities in the compressed air can contaminate the product, causing changes in color, taste, and even bacterial exposure. The product may be contaminated.
Product recalls can occur due to microorganisms such as compressed air, which is formed in situ by drawing in and compressing surrounding air and contains water vapor and particulate matter (the atmosphere typically contains 140–150 million dust particles). More importantly, the filtration system used protects process equipment from larger particles such as water, oil, rust, and pipe scale, with a nominal size of 25 to 40 microns, and can also filter sub-micron contaminants that cannot be removed. Compressed air is typically filtered to remove particulate matter to levels below 0.02 mg/m3 (100% size <0.01 microns).
Moisture:
Every atmosphere contains some water vapor, and when air or gas is cooled past its saturation point, the point at which it can no longer hold water vapor, it condenses into liquid water in a compressed air or gas system. Condensed water must be removed using separators and traps. Moisture in compressed air used in manufacturing plants can cause problems with air systems, solenoid valves, and air motors.
This moisture washes away the lubricating oil, increasing rust and wear on moving parts. Moisture will adversely affect the color, adhesion, and appearance of compressed air paints. Corrosion of air or gas equipment due to moisture can cause false readings and cause the process to stall or stop. If these controls fail due to rust, scale, or plugged holes, it can result in product damage and costly downtime. Additionally, control equipment often fails when moisture in control lines freezes in cold weather. Compressed air systems must have a moisture content of less than 0.01%.
Viable Count:
Manufacturers may not be aware that microorganisms may be present in compressed air systems. When air supply systems operate, they can release contaminants that can adversely affect the product, including its performance, violating the requirements for sterility and aesthetics of the product. The condition and quality of supply air from a microbiological point of view remain unclear until microbiological studies are carried out. However, a simple air exposure microbiological test of compressed air lines will alert manufacturers to the different types of viable microorganisms that may be present.
Manufacturers of equipment classified as medical devices, pharmaceutical operations or aseptic filling continually evaluate the environmental impact of their products during the manufacturing process. This estimate typically includes the facilities, equipment, and personnel involved in the assembly process. Sampling includes, but is not limited to, surface sampling, particulate counting, water analysis and product testing. However, compressed air can be easily overlooked if it is not initially included in environmental monitoring protocols or identified by an experienced environmental scientist. In compressed air systems, the number of viable bacteria should be less than 100 CFU.