CADILA PHARMACEUTICALS LIMITED-Walk In Interview For Manufacturing/ QA/ QC/ Engineering/ Formulation on 9th Dec,23 @Ahmedabad
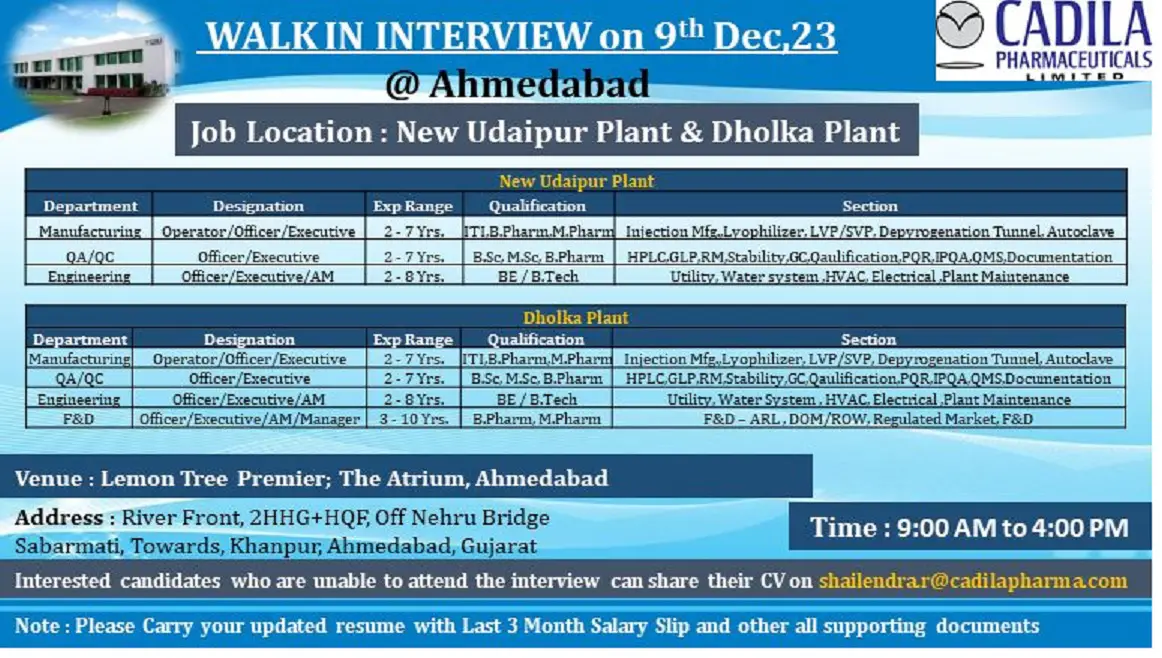
WALK-IN INTERVIEW on 9th Dec,23 @Ahmedabad
Job Location: New Udaipur Plant & Dholka Plant
New Udaipur Plant
Manufacturing-Operator/Officer/Executive/2-7 Yrs./ITI.B.Pharm.M.Pharm/Injection Mfg. Lyophilizer, LVP/SVP, Depyrogenation Tunnel. Autoclave
QA/QC-Officer/Executive/2-7 Yrs./B.Sc, M.Sc. B.Pharm/HPLC,GLP.RM.Stability,GC.Qaulification PQR,IPQA.QMS.Documentation
Engineering-Officer/Executive/AM-2-8 Yrs./BE/B.Tech/Utility, Water system HVAC, Electrical Plant Maintenance
Dholka Plant
Manufacturing –Operator/Officer/Executive/2-7 Yrs./ITI,B.Pharm.M.Pharm /Injection Mfg. Lyophilizer, LVP/SVP, Depyrogenation Tunnel, Autoclave
QA/QC–Officer/Executive/2-7 Yrs./ B.Sc. M.Sc, B.Pharm /HPLC GLP.RM.Stability GC Qaulification PQR.IPQA QMS.Documentation
Engineering–Officer/Executive/AM/2-8 Yrs./BE/B.Tech/Utility, Water System, HVAC, Electrical Plant Maintenance
F&D-Officer/Executive/AM/Manager/3-10 Yrs/ B.Pharm, M.Pharm./ F&D-ARL, DOM/ROW, Regulated Market, F&D
Venue : Lemon Tree Premier; The Atrium, Ahmedabad
Address: River Front, 2HHG+HQF, Off Nehru Bridge Sabarmati, Towards, Khanpur, Ahmedabad, Gujarat
Time: 9:00 AM to 4:00 PM
Interested candidates who are unable to attend the interview can share their CV on shailendrar@cadilapharma.com
Note: Please Carry your updated resume with Last 3 Month Salary Slip and other all supporting documents