Dasami Lab – Interview For QC/ Production/ Production (Documentation) on 7th Jan’ 2024
Dasami Lab – Interview For QC/ Production/ Production (Documentation) on 7th Jan’ 2024
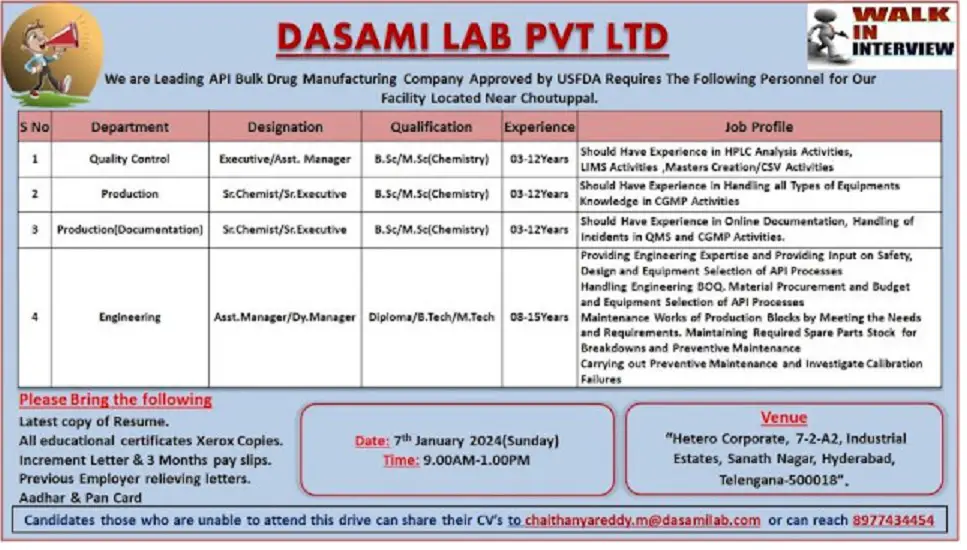
We are Leading API Bulk Drug Manufacturing Company Approved by USFDA Requires The Following Personnel for Our Facility Located Near Choutuppal.
1) Department: Quality Control
Designation: Executive/Asst. Manager
Qualification: B.Sc/ M.Sc(Chemistry
Experience: 03-12 Years
Job Profile: Should Have Experience in HPLC Analysis Activities, LIMS Activities, Masters Creation/CSV Activities
2) Department: Production
Designation: Sr.Chemist/ Sr. Executive
Qualification: B.Sc/ M.Sc(Chemistry
Experience: 03-12 Years
Job Profile: Should Have Experience in Handling all Types of Equipments Knowledge in CGMP Activities
3) Department: Production (Documentation)
Designation: Sr. Chemist/ Sr. Executive
Qualification: B.Sc/ M.Sc(Chemistry
Experience: 03-12 Years
Job Profile: Should Have Experience in Online Documentation, Handling of Incidents in QMS and CGMP Activities.
4) Department: Engineering
Designation: Asst. Manager/Dy. Manager
Qualification: Diploma/ B.Tech/ M.Tech
Experience: 08-15Years
Job Profile: Providing Engineering Expertise and Providing Input on Safety, Design and Equipment Selection of API Processes Handling Engineering BOQ. Material Procurement and Budget and Equipment Selection of API Processes
Maintenance Works of Production Blocks by Meeting the Needs and Requirements. Maintaining Required Spare Parts Stock for Breakdowns and Preventive Maintenance Carrying out Preventive Maintenance and Investigate Calibration Failures
Date: 7th January 2024(Sunday)
Time: 9.00AM-1.00PM
Venue: “Hetero Corporate, 7-2-A2, Industrial Estates, Sanath Nagar, Hyderabad, Telengana-500018”.
Please Bring the following
Latest copy of Resume.
All educational certificates Xerox Copies.
Increment Letter & 3 Months pay slips.
Previous Employer relieving letters.
Aadhar & Pan Card