V-Ensure Pharma- Walk-in Drive for Analytical Lab QC, Micro, Quality Assurance, Production, HR & ADMIN department 21st January 2024
V-Ensure Pharma- Walk-in Drive for Analytical Lab QC, Micro, Quality Assurance, Production, HR & ADMIN department 21st January 2024
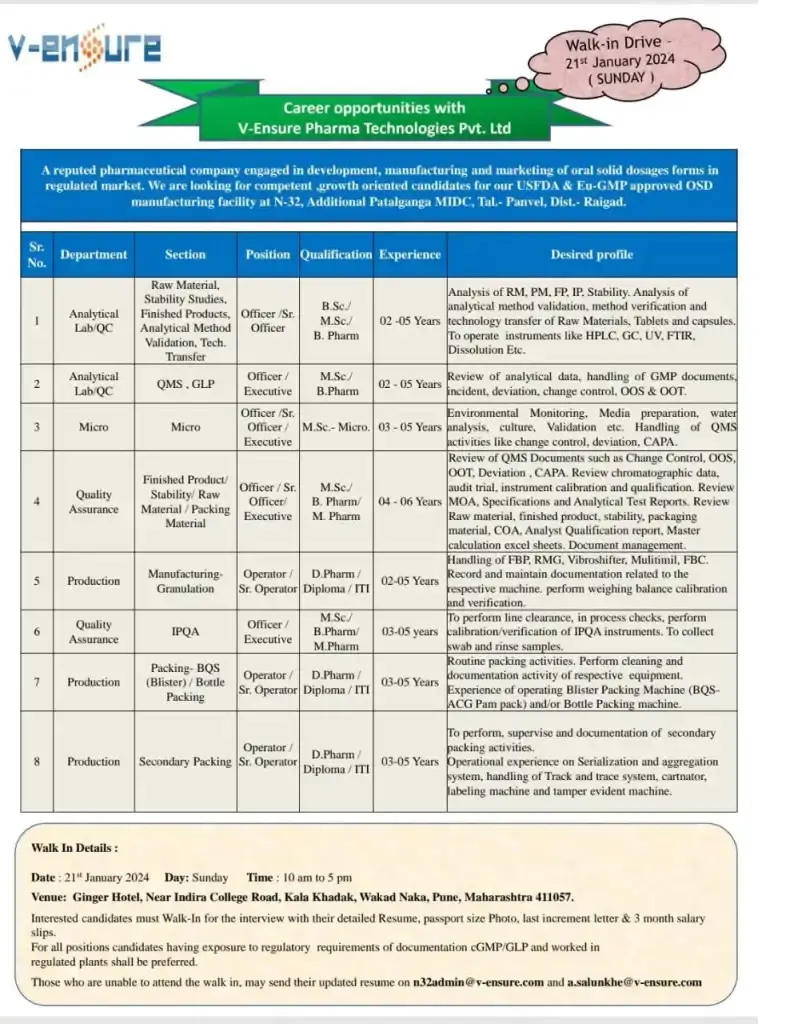
A reputed pharmaceutical company engaged in development, manufacturing and marketing of oral solid dosages forms in regulated market. We are looking for competent growth oriented candidates for our USFDA & Eu-GMP approved OSD manufacturing facility at N-32, Additional Patalganga MIDC, Tal. Panvel, Dist.- Raigad.
Walk-in Drive 21st January 2024 (SUNDAY)
Department: Analytical Lab QC, Micro, Quality Assurance, Production, HR & ADMIN
Position: Operator/ Sr. Operator/ Officer/ Sr. Officer/ Executive/ Apprentice
Qualification: D.Pharm/ Diploma/ ITI/ B.Sc./ M.Sc/ B. Pharm/ M.Pharm/ BMS/BBA/ MMS/MBA with HR Specialization
Experience: 02-06 Years
Date: 21 January 2024
Day: Sunday Time: 10 am to 5 pm
Venue: Ginger Hotel, Near Indira College Road, Kala Khadak, Wakad Naka, Pune, Maharashtra 411057.
Interested candidates must Walk-In for the interview with their detailed Resume, passport size Photo, last increment letter & 3 month salary slips. For all positions candidates having exposure to regulatory requirements of documentation cGMP/GLP and worked in
regulated plants shall be preferred.
Those who are unable to attend the walk in, may send their updated resume on n32admin@v-ensure.com and a.salunkhe@v-ensure.com