Operation, Cleaning and Overprinting of Semi- Automatic Carton Overprinting Machines
Objective
- To lay down a procedure for Operation, Cleaning and Overprinting of Semi-Automatic Carton Overprinting Machines.
Scope
- This Standard Operating Procedure is applicable for Operation, Cleaning and Overprinting Record of Semi-Automatic Carton Overprinting Machine & Semi-Automatic Carton Overprinting Machine.
Responsibility
- Packing Material Store and production personnel shall be responsible to follow the procedure mentioned in this SOP.
- Packing Material Store In-charge and production supervisor shall be responsible for implementation of the procedure mentioned in this SOP.
- Accountability
- Packing Material Store In-charge, Production Head, QC Head & QA Head shall be accountable for the implementation of this SOP.
Abbreviations and Definitions
- SOP : Standard Operating Procedure; a document where step by step instructions are cited to serve as support for methods or manners of fulfilling a function or functions reliably and consistently.
- QA : Quality Assurance
- HOD : Head of Department
- PM : Packing Material
- QC : Quality Control
- Ltd. : Limited
- B.No : Batch Number
- ID : Identification
- Mfg : Manufacturing
- Exp : Expiry
- A.C. : Alternate Current
Procedure
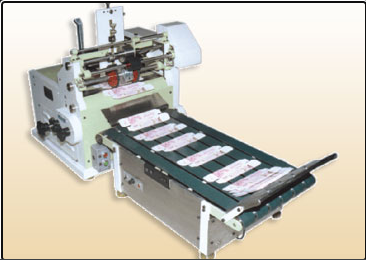
Salient features of Machine:
- C. Drive for Fine Operation
- Adjustment of Lock Bottom Carton
- Counting Arrangement
- Die to setup the desired stamp
Adjustment and Operation:
- Set the stereo rubber on die with letters and figures properly to get the impression as per the required coding particulars.
- Apply the printing ink on the Lapping Roller and then adjust the printing block.
- Switch on the main supply in order to run the motor.
- Now place the carton under the printing block and press the paddle.
- Check the impression on the carton if not proper then check for the proper location of the carton and the stereo rubber on die.
- After getting proper impression mark the location in order to get uniformity.
- After adjusting and before starting operation of overprinting, the operator shall get the adjustment (setting) checked by the Store Personnel.
- Now start overprinting by placing the cartons at right place.
- This overprinting machine shall be used for overprinting of B. No., Mfg Date, Exp Date, and price on the inner or outer cartons.
Cleaning of the machine:
- Switch off the machine.
- The ink roller shall be cleaned with the help of ink reducer and thinner and finally shall be cleaned with the help of a piece of lint free cloth.
- Remove the figures and letters from the die and place them in the box meant for the purpose.
- The body of the machine shall be cleaned with the help of a piece of lint free cloth.
- CLEANED status label shall be affixed.
- Make sure that no packing material i.e. printed/unprinted shall remain present near the machine.
- Record regarding operation and cleaning of this overprinting machine shall be maintained in respective log book maintained for this purpose.
Overprinting (Coding) Record:
- The record regarding the particulars to be coded and setting of the machine for the particulars to be overprinted shall be entered by the store personnel in the log book maintained in this respect given in Annexure-I, which shall be checked by Store personnel and verified by production, QC and QA personnel.
Precautions:
- Before operating the machine grease/oil shall be applied to the parts which are required to be greased/ oiled.
- Before starting the operation of printing it shall be ensured that the machine is neat and
- clean and no material printed earlier is lying near the machine.
- The cartons used at the time of adjustment of overprinting shall be destroyed by cutting into pieces before starting the overprinting operation.
- The store personnel shall make entries of entire overprinting process simultaneously in the log book prepared.
- The overprinted material of a particular batch shall be kept separately and shall not be mixed with unprinted material or overprinted material of other batches/products.
- Whenever any abnormal sound is observed the operator shall immediately switch off the machine and look for the fault and, if needed, shall get the fault removed by the maintenance department.
- Before setting the Die with letters and figures the operator shall ensure the particulars given in the Coding Control Record sheet provided by the Production Department and verified by the concerned persons.
- After adjustment of the machine the operator shall get the printed specimen verified by Store Personnel, Production Personnel, QC personnel and QA Officer, before starting the process of overprinting.
Forms and Records (Annexures)
- Coding (Overprinting) record of Packing Material – Annexure – I
Distribution
- Master copy – Quality Assurance
- Controlled copies – Quality Assurance, Packing Material Store
- History
Annexure – I
Coding (Overprinting) record of Packing Material
| Prepared by | Checked by | Approved by | ||
| Deptt. Head | Head QA | |||
| Sign & Date | ||||
| Name | ||||
| Designation | ||||
| Effective Date: Next Review Date: |
| Date | Product coded | B. No. | Mfg. Date | Exp. Date | M.R.P. | Setting By (Operator) | Printed By (Operator) | Checked By Store | Verified By Production | Verified By QC | Verified By QA |
For more Pharma posts and updates click Here



