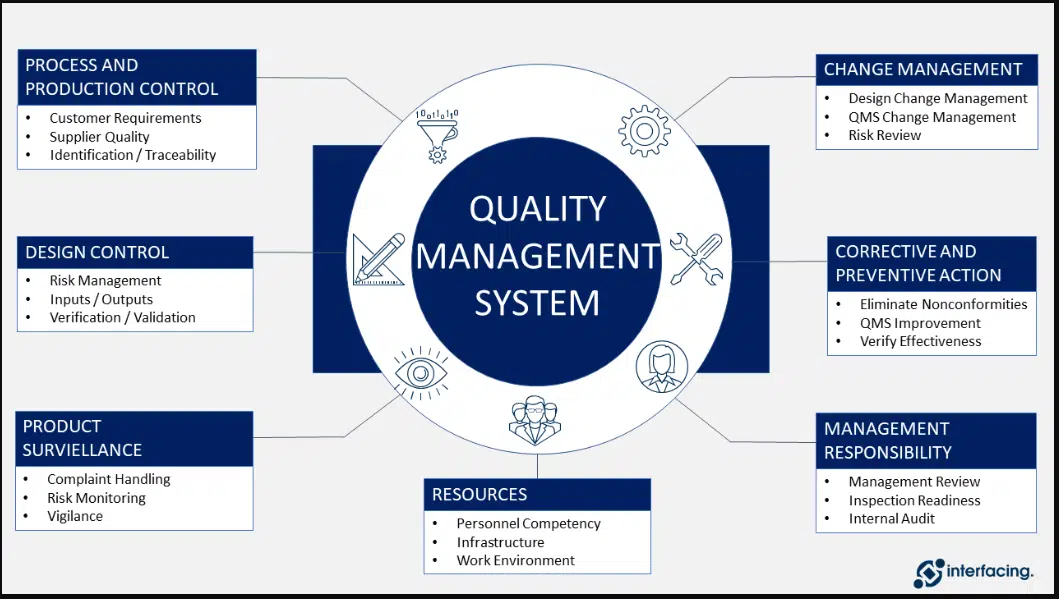
SOP for QMS Tools
SOP for QMS Tools Document Management –Review batch records, stability reports, validation reports, and associated documents required to comply with respective regulatory country GMP. Site Deviations – initiation, investigation, review, closeout, and maintenance for internal and external issues. Change Controls – initiation, review, closeout, and maintenance of all internal and external change controls. CAPA Management – manages…