Internal Audit in the Pharmaceutical Industry: Enhancing Governance, Risk Management, and Compliance
The pharmaceutical industry operates in a highly regulated and complex environment, with stringent quality and compliance requirements. To ensure the integrity of operations, enhance governance practices, manage risks effectively, and comply with regulatory standards, internal audit functions play a pivotal role. In this blog post, we will explore the importance of internal audit in the pharmaceutical industry, its objectives, key areas of focus, and the benefits it brings to pharmaceutical companies.
- The Significance of Internal Audit in the Pharmaceutical Industry
- Governance and Control: Internal audit serves as an independent and objective function within pharmaceutical companies, providing assurance to management and stakeholders that appropriate governance structures and internal controls are in place. By evaluating the effectiveness of internal control systems, internal auditors help identify control gaps, improve processes, and safeguard the organization’s assets and reputation.
- Risk Management: Pharmaceutical companies face numerous risks, including regulatory compliance, product quality, supply chain integrity, data security, and reputational risks. Internal audit functions assess and monitor these risks, helping organizations identify, evaluate, and mitigate potential risks. Through risk-based audits, internal auditors enable proactive risk management strategies, ensuring the company’s long-term sustainability.
Worst-Case in Cleaning Validation
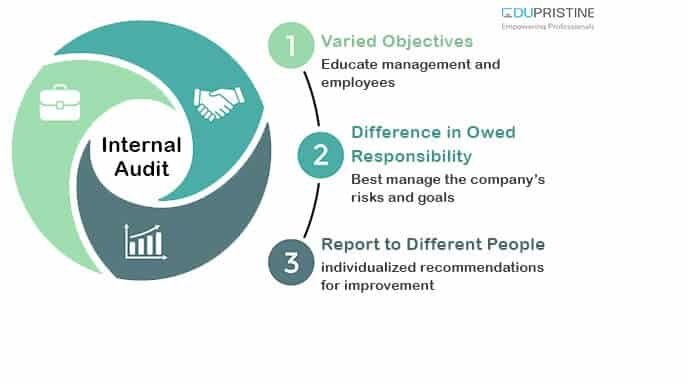
- Compliance with Regulations and Standards: The pharmaceutical industry is subject to a myriad of regulations and standards, such as Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and data privacy regulations. Internal audit plays a critical role in assessing compliance with these regulations and standards. By conducting compliance audits, internal auditors help identify non-compliant practices, implement corrective actions, and ensure adherence to regulatory requirements.
- Key Areas of Focus in Internal Audit
- Quality Management Systems: Internal auditors assess the effectiveness of quality management systems (QMS) within pharmaceutical companies. This includes evaluating processes and procedures related to document control, change management, deviation management, and corrective and preventive actions. Audits of QMS ensure that companies maintain the highest standards of quality and continuously improve their processes.
- Financial Controls: Internal audit functions also evaluate financial controls within pharmaceutical companies. This includes assessing the adequacy of financial reporting, asset management, cash management, and expense controls. By ensuring the integrity of financial operations, internal auditors contribute to accurate financial reporting and the prevention of fraud or misappropriation of funds.
- Data Integrity and Information Security: With the increasing digitization of pharmaceutical operations, data integrity and information security have become critical areas of focus. Internal auditors assess the controls and processes in place to safeguard data integrity, including data collection, storage, access controls, and validation processes. By addressing vulnerabilities and risks, internal audit functions help protect sensitive data and prevent data breaches.
- Supplier and Vendor Management: The pharmaceutical industry relies heavily on a network of suppliers and vendors. Internal auditors evaluate the effectiveness of supplier and vendor management processes, including selection, qualification, performance evaluation, and contract management. Audits in this area ensure that suppliers meet quality and compliance standards, reducing the risk of substandard materials entering the supply chain.
- Regulatory Compliance: Internal audit functions assist in ensuring compliance with regulatory requirements. Audits focus on evaluating adherence to GMP, GCP, pharmacovigilance, and other relevant regulations. By conducting audits, internal auditors help identify potential compliance gaps, implement corrective actions, and mitigate regulatory risks.
Benefits and Best Practices in Internal Audit
Auditing manufacturing processes in the pharmaceutical industry is a standard part of most quality system plans today. An internal audit helps assess the internal control of a system and measure the effectiveness of the quality system.
- Improved Risk Management: Internal audit provides valuable insights into risk identification, assessment, and mitigation. By conducting risk-based audits, internal auditors contribute to a proactive risk management culture, enabling companies to address potential risks before they escalate.
- Enhanced Operational Efficiency: Internal audit functions identify process inefficiencies and control weaknesses. By recommending process improvements and best practices, internal auditors help streamline operations, reduce costs, and enhance overall efficiency within pharmaceutical companies.
- Compliance and Regulatory Assurance: Internal audit plays a crucial role in ensuring compliance with regulatory requirements. By conducting regular compliance audits, internal auditors help companies stay abreast of regulatory changes, identify non-compliance issues, and implement corrective actions, mitigating regulatory risks.
- Stakeholder Confidence: An effective internal audit function builds stakeholder confidence by providing independent assurance on the company’s governance, risk management, and compliance practices. This, in turn, enhances trust among investors, regulators, customers, and the public.
- Continuous Improvement: Internal audit functions contribute to a culture of continuous improvement within pharmaceutical companies. By identifying areas for enhancement, recommending best practices, and monitoring the implementation of corrective actions, internal auditors facilitate ongoing process improvement and drive organizational excellence.
Conclusion of Internal Audit:
Internal audit is a critical function within the pharmaceutical industry, supporting governance, risk management, and compliance efforts. By evaluating internal controls, assessing risks, and ensuring compliance with regulations and standards, internal audit functions enhance operational efficiency, mitigate risks, and build stakeholder confidence. Through audits in key areas such as quality management systems, financial controls, data integrity, supplier management, and regulatory compliance, internal auditors contribute to the overall success and sustainability of pharmaceutical companies. Embracing best practices in internal audit and leveraging the expertise of internal auditors enable organizations to navigate the complex regulatory landscape, improve processes, and maintain the highest standards of quality, integrity, and compliance in the pharmaceutical industry.

