Jodas Expoim Pvt Ltd – interview for Production on 11th Feb 2024
Jodas Expoim Pvt Ltd – interview for Production on 11th Feb 2024
Jodas is a global, specialty, innovation-driven, emerging, generic pharmaceutical company, that is asserting itself among the worlds foremost pharmaceutical companies. We began our journey of growth in the year 2008 in India, and today Jodas is a modern, research-driven organization that has quality and affordability at the heart of its activities.
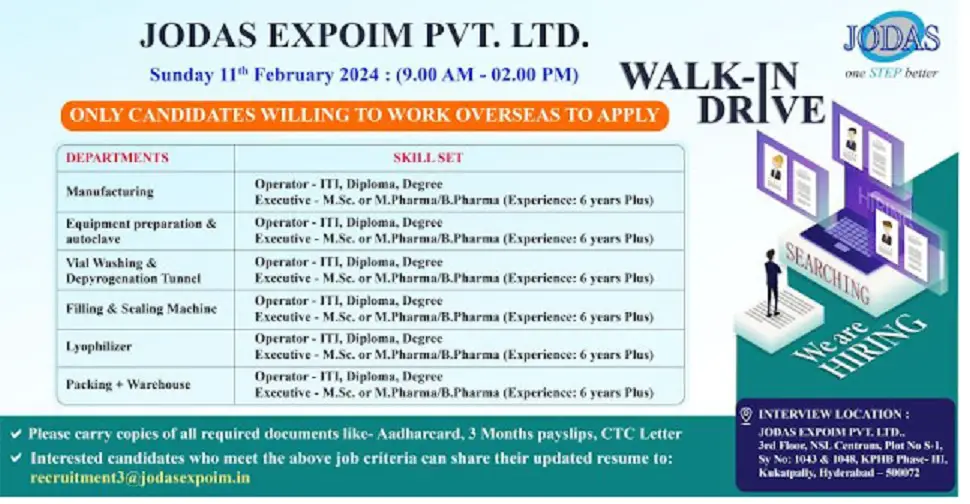
We are hiring for Production Injectables for Overseas Location(Only Male)
Experience : 4 – 10 Years
Job Description:
1. Manufacturing – Operator: ITI, Diploma, Degree, Executive – M.Sc. or M.Pharma/ B.Pharma (Experience: 6 years Plus)
2. Equipment preparation & Autoclave – Operator: ITI, Diploma, Degree, Executive – M.Sc. or M.Pharma/B.Pharma (Experience: 6 years Plus)
3. Lyophilizer – Operator: ITI, Diploma, Degree, Executive – M.Sc. or M.Pharma/B.Pharma (Experience: 6 years Plus)
4. Filling & Sealing Machine: B.Pharma/M.Pharma with a minimum of 6 years of relevant experience in Manufacturing, vial washing & Autoclave activities
5. Vial Washing & Depyrogenation Tunnel: Operator – ITI, Diploma, Degree, Executive – M.Sc. or M.Pharma/B.Pharma (Experience: 6 years Plus)
6. Packing + Warehouse – Operator: ITI, Diploma, Degree, Executive – M.Sc. or M.Pharma/B.Pharma (Experience: 6 years Plus)
Please carry your latest updated CV, last 3 months Pay slips& CTC breakup while coming to attend the interview.