SOP for QMS Tools
Document Management –Review batch records, stability reports, validation reports, and associated documents required to comply with respective regulatory country GMP.
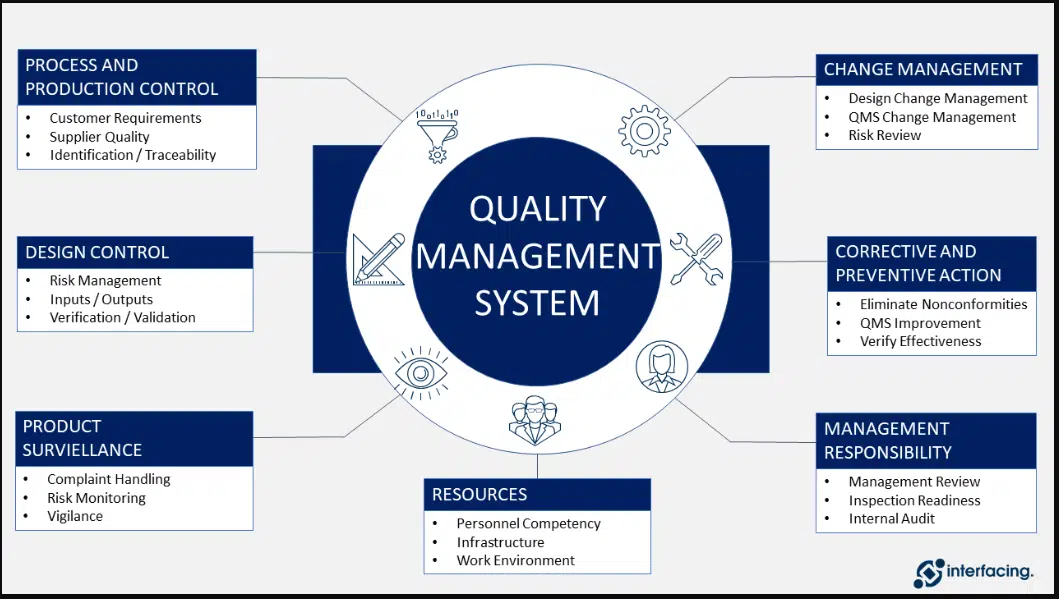
Site Deviations – initiation, investigation, review, closeout, and maintenance for internal and external issues.
Change Controls – initiation, review, closeout, and maintenance of all internal and external change controls.
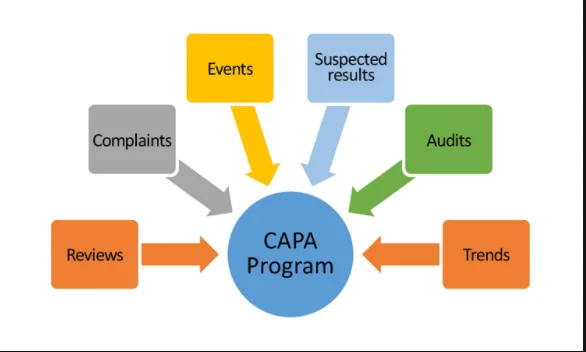
CAPA Management – manages CAPA and Investigation Program and ensures that CAPAs based on investigation of deviations, complaints, change controls, and audits are closed in a timely manner.
SOP Management – GMP-related SOPs, ensure they are followed, and meet current corporate and regulatory requirements.
Validation and Qualification Management – To review and approved design, validation, and implementation activities, Review validation protocols and reports, equipment/facility change requests, and calibration documentation for any new change control requests associated with plant operations.
Complaints – Provides QA support for complaint investigations and Ensures investigations are properly reviewed with respect to the root cause, CAPA requirements, and their evaluation.
Returned Goods Management – Manages the QA portion of the returns process per site SOP.
Warehouse Management- Co-ordinate warehouse QA activities for incoming inspection and release based on determining priorities and Participate in all recall actions as required with regards to inventory management and documentation.
Training Management –
Risk Assessment Management – Establish and maintain a risk management program and risk registrar tracking tool.
Self-Inspection and Inspection Readiness Program –
- Develop new procedures and update current procedures to ensure that they reflect current practices and pharmaceutical industry best practices.
- Manage assessments/gap analysis of the quality systems and design, develop and execute effectiveness measurement metrics and tools
- Review Track wise records for QA related activities such as UPD / Lab event / OOS / OOT / change control / CAPA and verification of action items