Storage, Retrieval, Control and Retention of all completed Documents
OBJECTIVE :
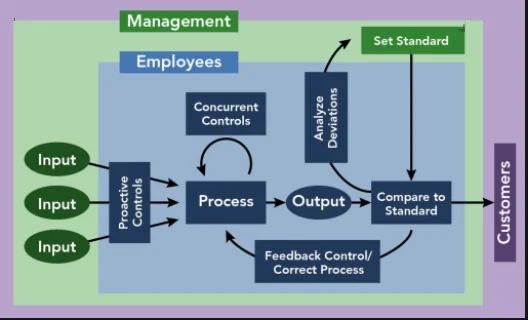
To outline a procedure for the storage, retrieval, control and retention of all completed documents, related to various functional areas to ensure security and traceability of completed records.
RESPONSIBILITY :
Concerned HOD or his designee to arrange for submitting respective completed documents related to their functional area.
Officer – Documentation (QA) for storage, retrieval, control and retention of all completed documents in Documentation cell.
HOD or his designee of the respective department to control the documents under their custody.
Head – Quality Assurance to ensure compliance.
PROCEDURE :
Location for storage of completed documents.
Completed documents generated in various functional areas shall be stored at the designated location as listed in Annexure-1.
Return of completed documents to Documentation Cell:
On the completion of a document, the ‘Return details’ for the particular document shall be entered in the respective control register (wherever applicable) by the concerned Head of the Department or his designee.
Officer-Documentation shall check the total number of pages of completed document and receive the same by signing under the ‘Received by’ column
Control and Storage of completed documents :
Officer-Documentation shall enter details of completed documents received into the Control Register of Completed Documents (Refer Annexure -2),except for Worksheets for Record of Analysis and BPRs. The return details of these documents shall be entered in Annexure -3 & 4 respectively.
Retrieval of completed documents :
Photocopy Issuance :
Officer-Documentation shall provide a copy of Product Development Report/Method Development Report/ process validation report to the concerned department for their future reference when the original copy is received.
If need arises, Photocopy of a completed document shall be issued against a request Authorised by Head-QA or his designee. Officer-Documentation shall provide the requested copies of the completed document and keep the record.
Access to Completed Documents :
Any person who intends to refer to a completed document shall record the details in “Completed documents access Register (Refer Annexure-5). Access shall be authorised by Officer-Documentation.
Retention of completed documents :
Documents are classified into three categories based on retention period and location of storage
Documents for perpetual retention.
The documents under this category shall be stored infinitely (hard copy or electronic copy) in Documentation cell until the life of the product.
Refer Annexure-1 for a detailed list of documents coming under each of these categories.
For a document not falling in the above classification, consult Head-QA or his designee.
Destruction of completed documents :
After completion of retention period the completed documents shall be destroyed by Officer-Documentation after authorization by Head of QA department or his designee.
The details of destruction shall be recorded in the respective Control Register of completed documents.
DOCUMENTATION
Specimen format of “Control Register of completed documents” – Annexure 2
Specimen format of “BPR Issue Register ” – Annexure 3.
Specimen format of “Register for Issue of Worksheets for Record of analysis ” – Annexure 4.
Specimen Format of “Completed Document Access Register” – Annexure 5.
ANNEXURE – 1
List of Documents for perpetual retention :
Location : Documentation Cell
Department : R&D
- Product Development Report.
- Stability Protocols for Exhibit batch.
- Process Validation Reports.
- Process Validation Protocol.
- Technology Transfer Reports
- Sampling Protocol
- Packaging Protocol
Department : Analytical R&D
- Analytical Method Validation Protocols.
- Analytical Method Development Report.
- Analytical Method Validation Registers.
- Analytical Method Validation Reports.
Department : Production
- Batch Production Records of Exhibit Batch.
Department : Quality Control
- Finished Product and Stability Record of analysis for Exhibit batches.
- Raw material/packaging material Record of analysis for materials (AR No.) used in Exhibit batches.
- Analytical records related to cleaning validation.
- Analytical Method Transfer qualification reports and related registers.
Department : Validation
- Master Validation Plan.
- Validation Protocols for all the equipments.
- HVAC Validation.
- Water validation protocols
- Compressed air validation protocols.
- Cleaning validation protocols.
Department : Regulatory
- Abbreviated New Drug Application
- Dossiers
- Annual reports, supplements and amendments
- Plant Master Files/Site Master File.
Department : Quality Assurance
- Stability Protocols of Validation / commercial batches.
- Vendor Qualification files
ANNEXURE – 2
CONTROL REGISTER FOR COMPLETED DOCUMENTS
| S No. | Document No. | Title | No. of pages | Received on | To be retained upto | Destruction details | ||||
| Authorised by | Destroyed by | Destroyed on | ||||||||
|
|
| |||||||||
| REGISTER FOR ISSUE AND CONTROL OF BPR | ||||||||||||||||||
| PRODUCT : | ||||||||||||||||||
| QUALITY ASSURANCE | ||||||||||||||||||
| Batch Details | Issue Details | Additional issue details | Total No. | Receiving details | Te be | Destruction details | ||||||||||||
| S No. | Request No. | Batch No. | MPR No. | No. of | Received | No. of | Received | No. of | Received | of pages | No. of pages | Total pages | Received | retained | Authorised | Destroyed | Destroyed | |
| pages (A) | by / Date | pages (B) | by / Date | pages (C) | by / Date | D = A+B+C | As issued (D) | Extra*(E) | (F) = (D+E) | by/Date | upto | by | by | on | ||||
| ISSUE AND CONTROL OF WORKSHEETS FOR RECORD OF ANALYSIS | |||||||||||||||||||
| Issue | AR No./ | Title | Issue Details | Additional issue details | Total | Return Details | To be | Destruction details | |||||||||||
| Date | Batch No. | No. of pages | Received | No. of pages | Received | No.of pages | Received | No.of pages | Received | Pages | Worksheet | Other | Received | retained | Authorised | Destroyed | Destroyed | ||
| issued | by/Date | issued | by/Date | issued | by/Date | issued | by/Date | issued | pages | pages | by/Date | upto | by | by | on | ||||
| A | B | C | D | E = A+B+C+D | |||||||||||||||
| COMPLETED DOCUMENTS ACCESS REGISTER | |||||
| Date | Document | Document | Purpose | Signature | Authroised |
| No. | Title | by | |||
| Completed Document | |||||||
| Issue request | |||||||
| QUALITY ASSURANCE | |||||||
| Document Name : | |||||||
| Document No. : | |||||||
| Requested by : | Date : | ||||||
| Purpose : | |||||||
| Authorised by : | Date : | ||||||
| Issued by : | Received by : | Date : | |||||
| No. of Pages : | |||||||
| Returned by : | Received by : | Date : | |||||
| No. of Pages : | |||||||