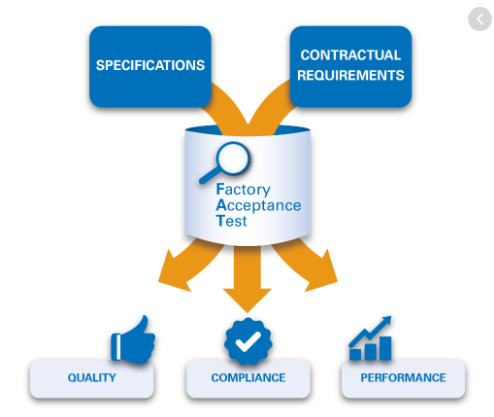
Factory Acceptance Test for Blister Pack Machine
Factory Acceptance Test for Blister Pack Machine machine name : BLISTER PACK MACHINE. Client Name & address : M/S BLISCARE MACHINES, MUMBAI. document name : factory acceptance test PROTOCOL no. : Revision no. : MACHINE SR. NO. : Table of Contents 1.… SYSTEM DESCRIPTION & SALIENT FEATURES :……………………………………………………………. 2.… SYSTEM CHECKS :…………………………………………………………………………………………………………… 2.1 Physical Check … Read more