Quality Management Review
Objective
- To lay down a procedure for Quality Management.
Scope
- This Standard Operating Procedure shall apply to formulation plant of Pharma Company.
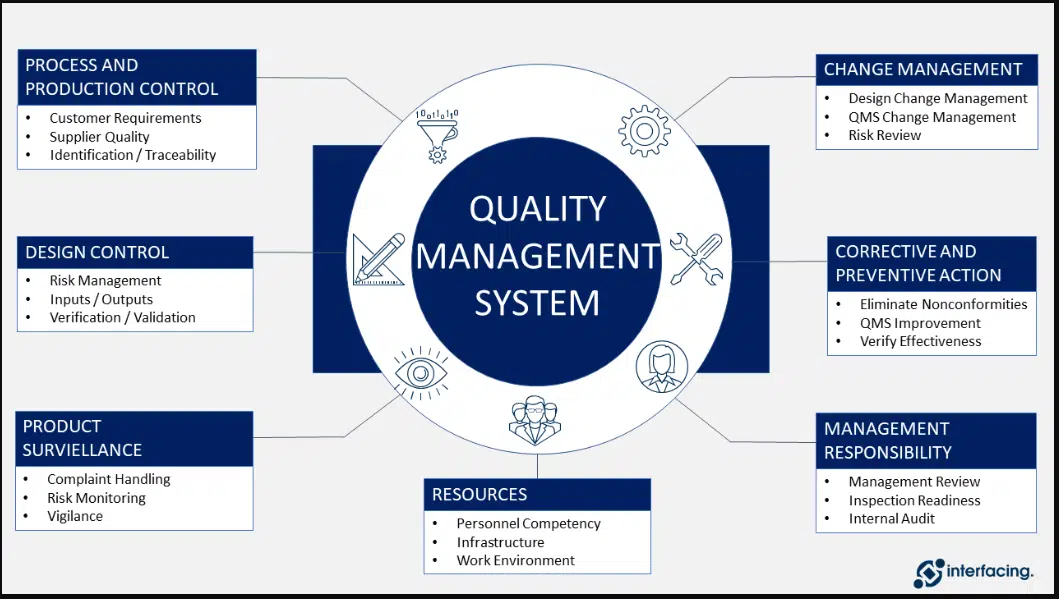
Responsibility
- Head Quality Assurance shall be responsible for compliance of this SOP.
- Heads of Production, QC, Stores, Engineering, and Human Resource etc. shall be responsible for implementation and compliance of the SOP.
Accountability
- QA Head / designee shall be accountable for implementation of this SOP.
Abbreviations and Definitions
- SOP : Standard Operating Procedure
- STP : Standard Test Procedure
- QA : Quality Assurance
- QC : Quality Control
- CAPA : Corrective and Preventive Action
- NCR : Nonconformance.
Procedure
- Quality Management Review shall be done by Quality Head.
- Quality Management shall provide the framework for implementing the quality procedures, check the suitability, adequacy and effectiveness of the quality system, continuous improvement and risk management.
- Following points shall be discussed under Quality Management review meeting; points are indicative only, the review may not be limited to these points.
- Batch Manufacturing records and other Production records.
- NCR, Deviations, CAPA and change control.
- System failures in the manufacturing process.
- SOPs, STPs and Specifications etc.
- Product complaints and Product recalls
- Internal Audits/Quality system
- Plan Vs. Actual
- Availability of materials as per the plan
- Capacity Utilization
- Cost effectiveness
- Manpower Utilization
- Preventive maintenance of critical equipments
- New initiatives for continuous improvement
- Operation and Quality Review meetings shall be organized at least once in every quarter of a calendar year.
- Meeting shall be concluded with possible solutions for all issues raised in meeting.
- The Recordings of the Quality Management meetings are confidential in nature and shall not be available to external agencies/ Auditors. The Selective information may be made available based upon the discretion of the company management.
Forms and Records (Annexures)
- Not Applicable
Distribution
- Master Copy – Quality Assurance
- Controlled Copies – Quality Control, Quality Assurance, Stores, Production, Engineering, Human Resources
History
| Date | Revision Number | Reason for Revision |
| I | New SOP |