SOP on handling of rinse and swab sample
Objective:
- To lay down the procedure for handling of rinse and swab sample.
Scope:
- This procedure is applicable for handling of rinse and swab sample in QC Department
Responsibility:
- Chemist or above of QC department
- Executive / nominee of QC department
Accountability:
- Head – Quality Control department
Procedure:
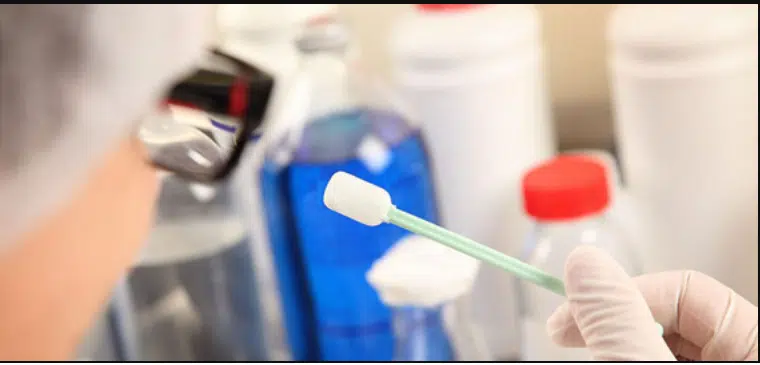
- In-process Quality Assurance personnel shall collect the sample of rinse or swab sample and handover the sample to QC department along with sample intimation slip as per annexure No. A03 filled with all details.
- Quality Control chemist or above shall record information of rinse and swab sample in rinse and swab sample inward register as per Annexure No. A01
- AR No. of rinse and swab sample shall be generated as per SOP .
- Quality Control Executive / Nominee shall allocate the sample to chemist for analysis.
- Quality Control Chemist shall perform the analysis as per standard test procedure of respective product, and record the raw data in continuation sheet.
- After completion of analysis, Executive / Nominee shall review the results and shall send to Head QC for final approval.
- After approval from QC Head one signed copy of rinse and swab intimation slip shall be send to IPQA section.
- If results are found out of specification, then (SOP on handling investigation and reporting of out of specification results) shall be followed for necessary action.
For Other Pharma Related Post and Updates Click Here
List of Annexure / Formats:
| Sr. No. | Format Title | Annexure Number |
| 1 | Rinse and swab Sample Inward Register | A01 |
References (if any).
Reason for Revision.
- Not Applicable due to new SOP.
Abbreviations:
- SOP : Standard Operating Procedure
- QC : Quality Control
- SG : Sub – General
- SP : Specific
- QA : Quality Assurance
- IPQA : In-process Quality Assurance
- AR.No. : Analytical Reference Number
Annexure 1
Rinse and swab Sample Inward Register
| Sr. No. | Date | Type of sample(Rinse / swab) | Name ofEquipment | Name ofprevious product | Previous productbatch No. | Equipmentto be used for | Sampled By | A.R.No. | Date of analysis | Analysed By | Status | Remarks | ||



