HPLC System Software Validation: Ensuring Accuracy and Reliability
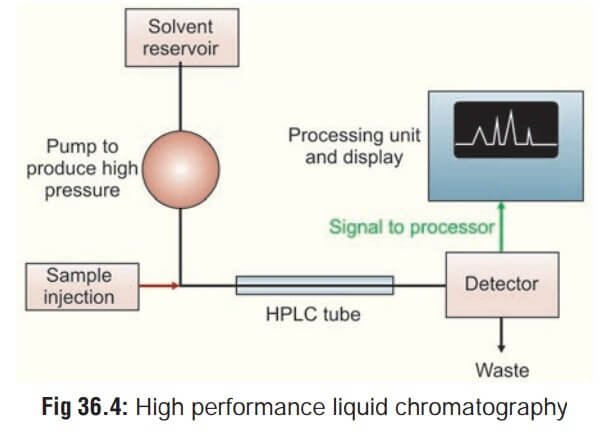
High-Performance Liquid Chromatography (HPLC System) is a widely used analytical technique in various industries, including pharmaceuticals, biotechnology, environmental analysis, and food and beverage. It allows for the separation, identification, and quantification of chemical components in complex mixtures. While the hardware components of an HPLC system play a vital role in achieving accurate and reliable results, the software that controls and manages these systems is equally important.
HPLC system software validation ensures that the software functions as intended, maintains data integrity, and complies with regulatory requirements. This blog post explores the significance of HPLC system software validation, its key components, and the best practices for successful implementation.
Understanding HPLC System Software
Before delving into software validation, it’s crucial to understand the role of HPLC system software in controlling and managing chromatographic processes. The software acts as the interface between the user and the hardware components, providing functionalities such as method development, instrument control, data acquisition, and analysis.
Modern HPLC system software offers a range of features, including customizable user interfaces, automated data processing, calibration and standardization tools, and audit trails for data traceability. These software packages are designed to enhance efficiency, accuracy, and reproducibility in analytical workflows. However, to ensure their reliability, it is essential to validate the software through a systematic and rigorous process.
Worst-Case in Cleaning Validation
Importance of HPLC System Software Validation
HPLC system software validation is critical for several reasons. Firstly, it ensures that the software performs as expected, minimizing the risk of errors or inconsistencies in data analysis and interpretation. By validating the software, users gain confidence in the accuracy and reliability of their results, enabling informed decision-making.
Secondly, regulatory agencies, such as the Food and Drug Administration (FDA), European Medicines Agency (EMA), and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have specific guidelines and requirements for software validation in regulated industries. Compliance with these regulations is crucial for obtaining regulatory approvals and maintaining data integrity.
Furthermore, HPLC system software validation aids in the identification and mitigation of potential risks associated with software malfunctions or deviations from expected performance. This proactive approach helps prevent issues that could lead to costly rework, product recalls, or compromised patient safety.
Components of HPLC System Software Validation
HPLC system software validation comprises several key components, each contributing to the overall assessment of software performance. The following are essential elements of a comprehensive validation process:
- User Requirement Specification (URS): The URS defines the specific requirements of the software based on user needs, including functionalities, performance criteria, and regulatory compliance. It serves as a foundation for the validation process.
- Installation Qualification (IQ): IQ ensures that the software is installed correctly and meets all the hardware and software requirements. This includes verifying system compatibility, installation procedures, and documentation.
- Operational Qualification (OQ): OQ evaluates the software’s performance under normal operating conditions. It involves testing system functionalities, instrument control, data acquisition, and data processing. OQ also includes verifying software configurations, security settings, and audit trail functionality.
- Performance Qualification (PQ): PQ assesses the software’s performance over an extended period, typically using real-world samples and complex analytical methods. It involves conducting method validation, system suitability testing, and evaluating data accuracy, precision, and linearity.
- Change Control: Change control ensures that any modifications or upgrades to the software are properly documented, evaluated, and validated. This process prevents unforeseen consequences that may affect data integrity or regulatory compliance.