Handling of Out of Specification Results
1.0 OBJECTIVE: To lay down a procedure for handling of Out-of-Specification (O.O.S.) test results generated during the testing.
2.0 SCOPE : These procedures are applicable to all QC testing of raw materials, finished product, and stability samples.
3.0 RESPONSIBILITY : Analyst/ Quality Assurance Manager
4.0 ACCOUNTABILITY : Quality Assurance Manager
5.0 PROCEDURE:
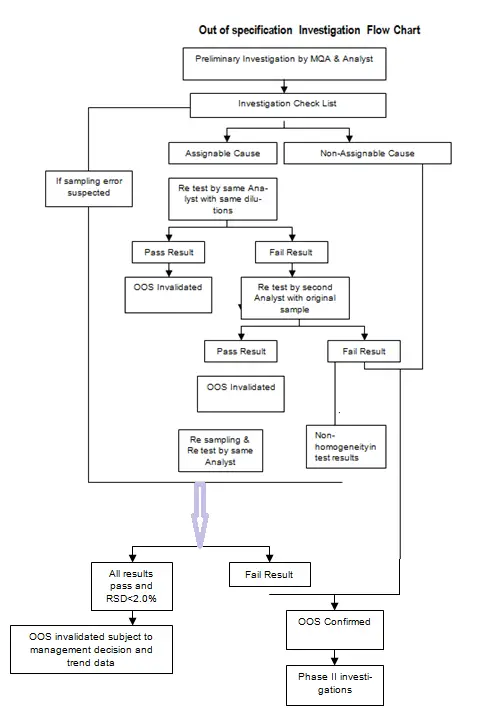
5.1 All out-of-specification test results (i.e. suspected test results that fall outside the established specifications or acceptance criteria) shall be investigated.
5.2 When an Out-Of-Specification test result is generated/ suspected, the analyst shall inform to the QA Manager immediately about the test results.
5.3 OOS number shall be allocated in the following manner. OOS/YY/XXX, where OOS represent the Out of specification, YY indicates the last two digits of the year & XXX indicates the serial number from 001 to 999. Enter the details in Log Book for out of specification results as per Annexure –I.
5.4 QA Manager will assess the data based on investigation to ascertain if the results can be attributed to laboratory error, or whether the results indicate problems in the manufacturing process.
5.5 Accordingly, the investigation Phase I and/ or II (Phase-I and II investigation relates to laboratory error and investigation of problems in manufacturing process, respectively) will be decided.
Phase-I Investigation
5.6 Both analyst and QA Manager are responsible for completing the Phase-I investigation (Annexure-II).
5.7 The first part of this investigation shall be an initial assessment of the accuracy of laboratory’s data (i.e. finding of an assignable cause, as per Annexure-I), before any test solutions are discarded.
5.8 Re-testing on the retained test solutions/ standard solutions shall be performed immediately by the same analyst who performed the original test, (For this purpose, all original test solutions shall be preserved until calculation of test results is done).
5.9 If clear evidence of laboratory error exists and the cause of O.O.S. can be assigned to this laboratory error (like sample preparation, analytical method followed, equipment malfunctions etc.) In this case the original O.O.S result may be invalidated. The cause of O.O.S. shall be documented, and the subject batch may be released.
5.10 If the initial O.O.S. results are not assigned to original solutions of test preparations, analyst error, equipment malfunction etc., retest from the original portions of sample drawn may be performed after due authorization by QA Manager. Re-testing shall be done (in triplicate) by the analyst other than the one who has performed original test.
5.11 If none of the three results obtained is out of specification, results may be averaged. The RSD of the triplicate results should not be more than 3%.
5.12 If such retest results meet the specifications, cause of original O.O.S. must be investigated and documented. If the cause can be assigned to a definite Analytical error with specificity to certain area of retesting, and the O.O.S. is not related to manufacturing process, then original O.O.S. may be invalidated.
5.13 Depending upon the nature of Analytical error, a review shall be performed, if such errors have past history. Accordingly, review of Analytical method, equipment calibration or re-training / revalidation of Analyst shall be performed.
5.14 Complete review of this investigation shall be documented, to minimize reoccurrence of such incidents in future.
5.15 Re-sampling may be permitted, by Manger-QA, only if, retest data from same original portion of the sample have been evaluated and the subsequent investigation gives possible evidence in respect of errors in sampling. (e.g. Non-representative ness of the original sample is indicated by wide variation in results from several aliquots of the original sample).
5.16 Re-sampling, if permitted, shall be performed using the same sampling procedure.
5.17 Evaluation of data from re-sampled material shall be performed to determine if original sample was not representative. If there is adequate evidence to support error in initial sampling (like contamination from sampling tool or wrong identification or sampling from damaged container etc.), and if it is established that original O.O.S. result was due to faulty sampling or errors in sampling procedure, then only the original O.O.S. result can be invalidated.
5.18 Testing of re-sampled aliquot shall be performed by the same analyst who performed the original analysis and with the same sampling method, in triplicate to establish new basis for batch release.
5.19 If 3 such data meets specifications individually and RSD of data is within 3% limit, original O.O.S. result is invalidated, and batch may be considered for release. While taking such decision, it should adequately be established that there are no failures in Manufacturing Process.
5.20 If out-of-specification test results are generated in Disintegration, Dissolution, and Uniformity of dosage units tests, and the cause is non-assignable, then applicable Pharmacopoeia (USP/BP/EP/IP) procedure shall be followed.
5.21 All steps leading to O.O.S. investigations shall be documented and review of each failure shall be correlated to occurrence of similar failures in the past. An action plan shall then be prepared, depending upon the nature of failure found which caused O.O.S..
5.22 If no assignable cause is found till this stage, the original results shall be considered VALID and the subject batch shall be REJECTED.
5.23 For further detailed investigation into O.O.S. result, Phase II investigation shall be initiated to determine the cause of Failure in the manufacturing process.
Also read : PROCEDURE FOR OPERATION OF UNIT DOSAGE SAMPLER
Phase – II Investigation
5.24 It should be carried by a Group consisting of, Manager QA, Manager Production and Manager Pharma Technology & Development (PTD) (If required). (Annexure – II)
5.25 This phase shall also take into account the complete evaluation of Batch Process Records related to the subject batch.
5.26 It shall cover complete review/ evaluation into potential manufacturing causes of the O.O.S result and it’s impact on preceding or succeeding batches of this product.
5.27 Trend analysis of previous batches shall be reviewed, if there is any evidence to indicate Failures prior to observation of O.O.S. result and also to assess the impact of Failure on previous batches. (Phase II investigation may also be performed parallel to Phase I investigation)
5.28 Investigation and resolution of all O.O.S. test results is a priority matter and hence Phase-I investigation should be completed within 72 hours. In case of a confirmed O.O.S. Result, Phase II investigation to identify the cause of Failure should be completed within 30 days and an Action Plan (to prevent the re-occurrence of the similar test results) should be circulated to all concerned departments.
ANNEXURE:
OOS log book – annexure I
Phase –I & II investigation report – annexure II
PHASE-I & II INVESTIGATION REPORT
PHASE- I INVESTIGATION REPORT
OOS No. :
Name of Material :
Batch No./A.R. No. :
Mfg. Date :
Exp. Date :
Specification No. & Revision No. :
Test Procedure No. & Revision No. :
Test in which O.O.S. result is found :
Result :
Specification Limit :
Date of Analysis :
Analyst (Name / Signature / Date) :
Investigation Started on :
- Investigation Checklist
| Sr.No | Parameters | Status | ||
| 01 | Sample appearance | Correct | Incorrect | NA |
| 02 | Weighing Errors | Yes | No | NA |
| 03 | Calculation Errors and raw data | Yes | No | NA |
| 04 | Completeness of raw data | Yes | No | NA |
| 05 | Validity of instrument calibration | Yes | No | NA |
| 06 | Instrument /Equipment malfunctioning | Yes | No | NA |
| 07 | Spilling or incomplete transfer of dilutions / sample | Yes | No | NA |
| 08 | Any broken or un cleaned glassware used | Yes | No | NA |
| 09 | Dilution make up volume | Correct | Incorrect | NA |
| 10 | Validity of reference or working standard | Yes | No | NA |
| 11 | Validity /Correctness of reagents, test and volumetric solutions | Yes | No | NA |
| 12 | Chromatographic test condition / System suitability requirement as per Standard test procedures | Correct | Incorrect | NA |
| 13 | Techniques used in carrying out analysis | Correct | Incorrect | NA |
| 14 | Method used for test | Correct | Incorrect | NA |
| 15 | Analyst is trained to carry out the test | Yes | No | NA |
| 16 | Suitable environmental conditions to carry the test | Yes | No | NA |
| 17 | Is correct sampling procedure followed | Yes | No | NA |
| 18
| Any other observation or remarks | |||
2.0 Checklist (Sampling)
| S.No | Parameters | Observation |
| 01 | Sampling performed by | |
| 02 | Date of sampling | |
| 03 | Total number of containers | |
| 04 | Number of containers sampled | |
| 05 | Storage conditions (Temp./ Rh) | |
| 06 | Storage period | |
| 07 | Expiry date of product | |
| 08 | Physical observations of sample | |
| 09 | Packing details |
2.0 Was Assignable cause (s) found? Specify the cause(s)
- If cause is calculation error, mention the recalculated result.
3.0 If assignable cause is established to be the laboratory error :
- Re analysis of the original sample and same dilutions done by :
- Details of test results obtained :
Analyst (Signature/Date)
4.0 If cause is not assignable, re-testing from originally drawn sample:
- Whether such observations are noticed earlier Yes / No
(due to analyst, method, equipment etc.)
- Re testing : Permitted Not Permitted
- Re-testing authorized by :
QA Manager
(Signature & Date)
- Details of results observed after-re testing
| Test Replicate | Results |
| 1 | |
| 2 | |
| 3 | |
| Average | |
| RSD : NMT 3.0% (Original O.O.S. results not to be included) |
Analyst (Signature/ date)
- Any sampling error suspected – Yes No
- Re-sampling : Permitted Not Permitted
- Re-sampling authorized by :
QA Manager
(Signature/Date)
Details of results observed :
| Test Replicate | Results
|
| 1 | |
| 2 | |
| 3 | |
| Average | |
| RSD : NMT 3.0% (Do not include original O.O.S. results) |
Attach: Copies of all analytical findings and results, put references of analytical data also.
- Corrective action(s) if an Assignable cause(s) was found?
Conclusion of Phase-I Investigation :
- O.S. result is Invalidated/ Confirmed.
Investigation Report Completed by :
| Name | |||
| Signature |
| ||
| date | Analyst 1 | Analyst 2 | QA manager |
Final Decision of QA Manager :
——————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————————
Perform Phase II Investigation : Yes/No
Batch Status : APPROVED/ REJECTED
Name :
Sign & Date :
PHASE- II INVESTIGATION REPORT
(This format should be used for documentation of investigation)
OOS No. :
Name of Material :
Batch No./A.R. No. :
Mfg. Date :
Exp. Date :
Specification No. & Revision No. :
Test Procedure No. & Revision No. :
Test in which O.O.S. result is found :
Result :
Specification Limit :
Date of Analysis :
Analyst (Name / Signature / Date) :
Investigation Started on :
Investigation findings :
(Evaluation of the Batch Production Record)
1.0 Process Related
1.1 Whether standard master formula is adhered :
1.2 Whether standard manufacturing procedure is adhered :
and correct entries made in Batch Production Record
1.3 Whether specified and validated equipment is used :
1.4 Any deviation from cGMP – SOPs (give details) :
1.5 Whether any change in Blending time/drying time etc :
1.6 Any Human error (wrong wt. addition, wrong material :
addition, wrong sequence of addition)
1.7 Whether any spillage of material noticed during processing :
1.8 Any other deviation in the batch, if so, give details thereof :
1.9 Whether the Environmental conditions, specified for the :
process are followed.
1.10 Whether the storage container is suitably stored at the :
designated place, at different stages of the manufacturing.
Give details of the storage time at different stages.
1.11 Reconciliation of yield at various stages. If not meeting :
the standard, then state the reason thereof.
2.0 NON-PROCESS RELATED
2.1 Whether any malfunctioning of equipment is noticed :
2.2 Whether any power failure occurred for extended period during :
the manufacturing
2.3 Other observations (if any) :
3.0 Conclusion of Investigation (Brief here):
- If investigation reveals any process problem, then give report/comment of PRD Head*:
- Trend analysis of the previous batches*:
- Impact of O.O.S. test results on Preceding and Succeeding batches of the product*:
* Attach additional sheets, if required.
Investigation Report Completed by :
| Name | |||
| Signature |
| ||
| date | Manager – Production 1 | PRD Head (if applicable) | QA Manager |
Investigation Report Approved by :
Action Plan circulated : Yes/No
QA Head
Sign & date :
Annexure – I
LOG BOOK- OUT OF SPECIFICATION
| OOS Number | Name of the Product | Parameter | OOS Initiated By | OOS Generation Date | Investigation start Date | OOS Confirmed / Failure | Performed By | Checked By | Completion Date |




